Our main focus is still on ruthenium complexes; however, in recent years we have also started to explore rhenium [1–3]. Historically, rhenium primarily served as a chemical analogue for technetium, as it has similar chemistry and stable isotopes. This allowed researchers to study technetium-based radiopharmaceuticals without the risks and limitations associated with radioactive materials.
In recent decades, however, rhenium chemistry has gained popularity as an independent field of research thanks to its unique and remarkable properties. Rhenium exhibits multiple oxidation states, which enables the synthesis of a variety of compounds. In addition, it possesses valuable radioactive isotopes that can be used for therapeutic or diagnostic purposes (theranostics). Some rhenium compounds also have favourable photochemical properties in biological systems. They are non-toxic in the dark, but become highly active when exposed to light and are therefore suitable for phototherapy applications.
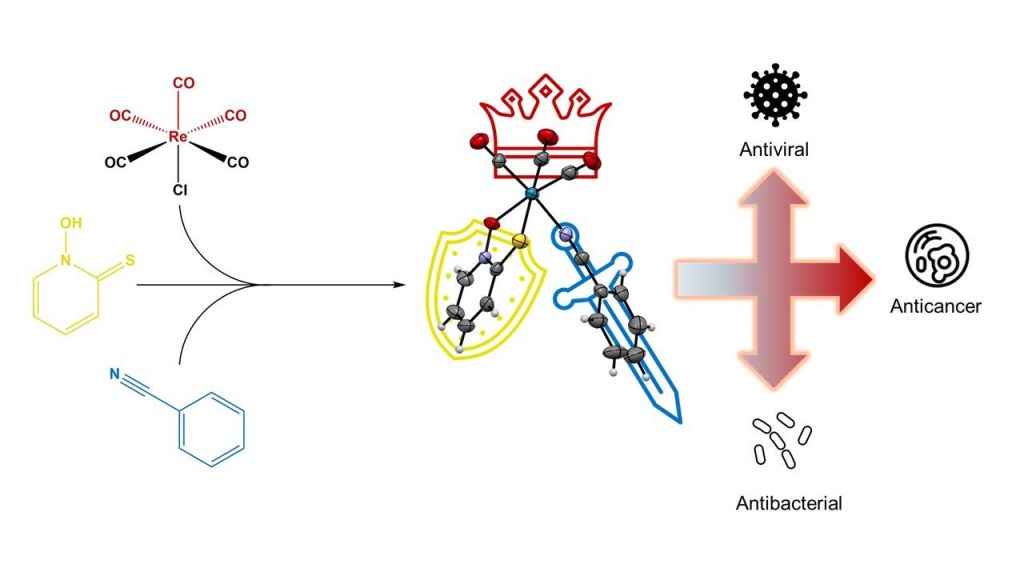
Recently, we reported the synthesis of the fac-tricarbonylrhenium(I) complex with a pyrithione ligand, which proved to be more challenging than initially expected [1]. During this study, we also identified several by-products and decomposition products, which we successfully characterised (see Scheme below). The stable fac-[Re(CO)3(pth)(benzonitrile)] complex was further investigated for its solution chemistry and evaluated for anticancer, antibiotic, and antiviral activities in collaboration with Professor Éva A. Enyedy’s research group.

Furthermore, in collaboration with Professor Janko Kos and Assistant Professor Ana Mitrović, we investigated the potential mechanism of action of inhibiting cysteine cathepsins, enzymes known to promote tumour growth. In addition, in partnership with Professor Stanislav Gobec and Assistant Professor Anže Meden, we conducted in silico studies to explore how this complex interacts with cathepsin B through docking and molecular dynamics simulations. The complex demonstrated promising anticancer activity against various cancer cell lines, antibiotic activity against different bacterial strains and notable antiviral activity against Herpes Simplex Virus 2. This is the first publication resulting from the doctoral research of Ph.D. student Uroš Rapuš.
More details can be found in the full publication that was published in ACS Omega:
1) U. Rapuš, T. Pivarcsik, A. Mitrović, J. Kljun, A. Bogdanov, G. Spengler, A. Meden, S. Gobec, J. Kos, E. Enyedy, I. Turel: Challenges in the preparation of a fac-tricarbonylrhenium(I) complex with pyrithione, its physico-chemical characterization and assessment of biological effects. ACS Omega, 10, 38272-38291, 2025.
https://doi.org/10.1021/acsomega.5c06647
2) T. Pivarcsik, M. A. Kiss, U. Rapuš, J. Kljun, G. Spengler, É. Frank, I. Turel, É. A. Enyedy: Organometallic Ru(II), Rh(III) and Re(I) complexes of sterane-based bidentate ligands: Synthesis, solution speciation, interaction with biomolecules and anticancer activity. Dalton Trans., 53, 4984-5000, 2024.
https://pubs.rsc.org/en/content/articlelanding/2024/dt/d3dt04138g
3) T. Pivarcsik, J. Kljun, S. Clemente Rodriguez, D. Cortéz Alcaraz, U. Rapuš, M. Nové, E. F. Várkonyi, J. Nyári, A. Bogdanov, G. Spengler, I. Turel, E. Enyedy: Structural and solution speciation studies on fac-tricarbonylrhenium(I) complexes of 2,2’-bipyridine analogues. ACS Omega, 9, 44601, 2024.