Russian chemist Dmitri I. Mendeleev (1834–1907) has formulated the periodic system of chemical elements (PSCE) in 1869. Frequently, this is considered as one of the greatest inventions in chemistry. The year 2019 marks the 150th anniversary of this discovery and has thus been proclaimed the “International Year of the Periodic Table of Chemical Elements (IYPT2019)” by the United Nations General Assembly and UNESCO.
Philately is my hobby and in the last years I have focused especially on chemical items. From time to time I prepare short articles for newspapers or journals on various topics and support them with philatelic materials (1-4). I have recently prepared a short contribution about the periodic table, Mendeleev and also about some lastly discovered chemical elements for Slovenian journal »Kemija v šoli in družbi« (Chemistry in school and society) (5). Therein I have involved selected philatelic items that are related to periodic table and some I present also below. I am sure that this year some new stamps, first day covers (FDC), seals, etc., will appear to celebrate IYPT 2019.
Figure 1-6 (from top to bottom):
It is understandable that most of stamps that show Mendeleev were issued in Russia (and previously in Soviet Union). To the best of my knowledge, the first stamps showing Mendeleev were issued in 1934 (100 years after his birth).
Fig. 1 shows one of these. The stamp issued to commemorate 100 years of periodic table of chemical elements discovery (1969) shows Mendeleev deep in thought (Fig. 2). Two chemical elements that represented some problems to Mendeleev are marked in red. Gallium was then not yet discovered but he has predicted its existence. It was discovered by French chemist Paul Emile Lecoq de Boisbaudran in 1875. In contrast, indium was already known but its relative atomic mass was not determined accurately. Non-accurately determined relative atomic masses were the reason that in the first versions of the periodic table some elements were placed in wrong positions which was later corrected in improved versions. Interestingly, also the available relative atomic mass of ruthenium, the most important element for our research group was not very precise at that time (104.4; correct value is 101.1).
A commemorative postcard (Fig. 3) showing the portrait of Mendeleev with a stamp and corresponding seal were issued in 1957 (50 years after his death).
A souvenir sheet from 1969 (100 years of PSCE discovery) shows the stamp with Mendeleev which is embedded in his signed and dated work paper (Fig. 4).
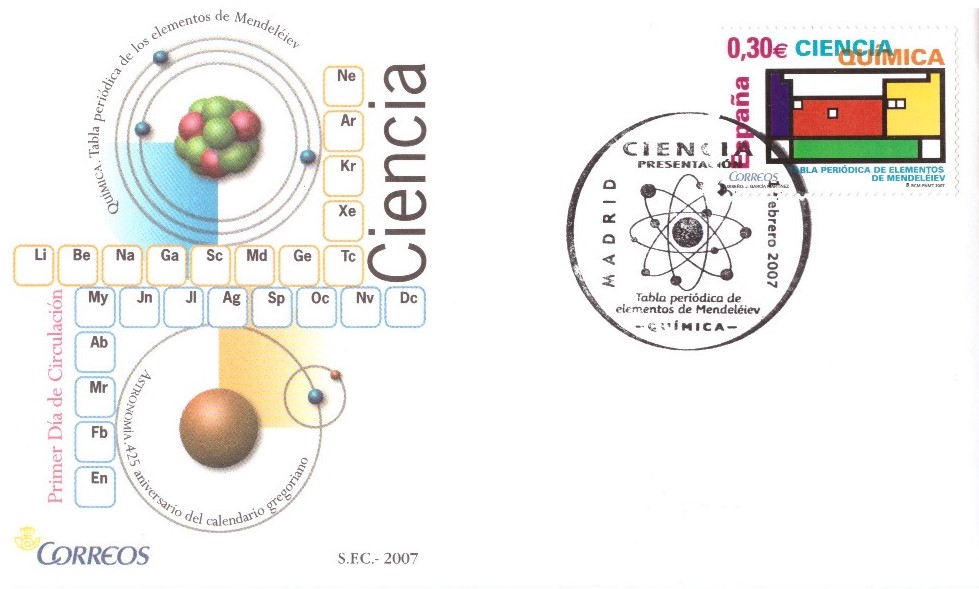
Spanish post has issued the first day cover in 2007 (hundred years after Mendeleev died) (Fig. 5). The corresponding stamp shows a colourful drawing of the periodic table which was inspired by the paintings of Piet Mondrian, the Dutch painter. Four smaller white squares in the periodic table represent elements that were predicted by Mendeleev (scandium, technetium, gallium, and germanium).
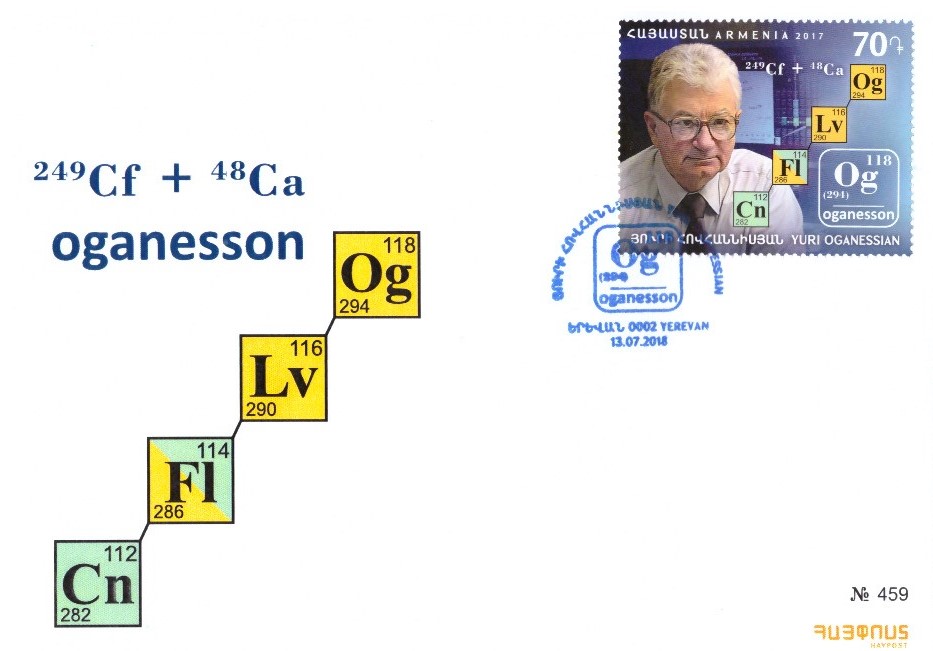
First day cover from Armenia shows Yuri Oganessian who is one of the leading nuclear physicists in the field of superheavy chemical elements (Fig. 6). He was involved in the preparation of several elements, among them is also the currently heaviest which is named oganesson (Og) in his honour. Artificial heavy elements are very unstable and the radioactive decay pathway of the isotope oganesson-294 is also shown on the stamp and cover. With the isolation of this element the 7th row of periodic table was completed (Z = 118). The methods of how to “synthesize” new chemical elements, how to verify and name them, as well as some speculations about possible new element discoveries in the coming years were nicely presented in the paper of Prof. J. Reedijk recently (5).
References:
- I. Turel, Acta Chim. Slov., 59, p. S94-S96 (2012).
- I. Turel, Acta Chim. Slov., 60, S115-118 (2013).
- D. Rabinovich, I. Turel, Philatelia chimica et physica, 35, 171-173 (2014).
- I. Turel, Kemija v šoli in družbi, nr. 1, p. 1-4 (2019).
- J. Reedijk, Row 7 of the periodic table complete: Can we expect more new elements; and if so, when?, Polyhedron, 2018, vol. 141, str. 1–4.