Turel Group and Rhenium
Our main focus is still on ruthenium complexes; however, in recent years we have also started to explore rhenium [1–3]. Historically, rhenium primarily served as a chemical analogue for technetium, as it has similar chemistry and stable isotopes. This allowed researchers to study technetium-based radiopharmaceuticals without the risks and limitations associated with radioactive materials.
In recent decades, however, rhenium chemistry has gained popularity as an independent field of research thanks to its unique and remarkable properties. Rhenium exhibits multiple oxidation states, which enables the synthesis of a variety of compounds. In addition, it possesses valuable radioactive isotopes that can be used for therapeutic or diagnostic purposes (theranostics). Some rhenium compounds also have favourable photochemical properties in biological systems. They are non-toxic in the dark, but become highly active when exposed to light and are therefore suitable for phototherapy applications.
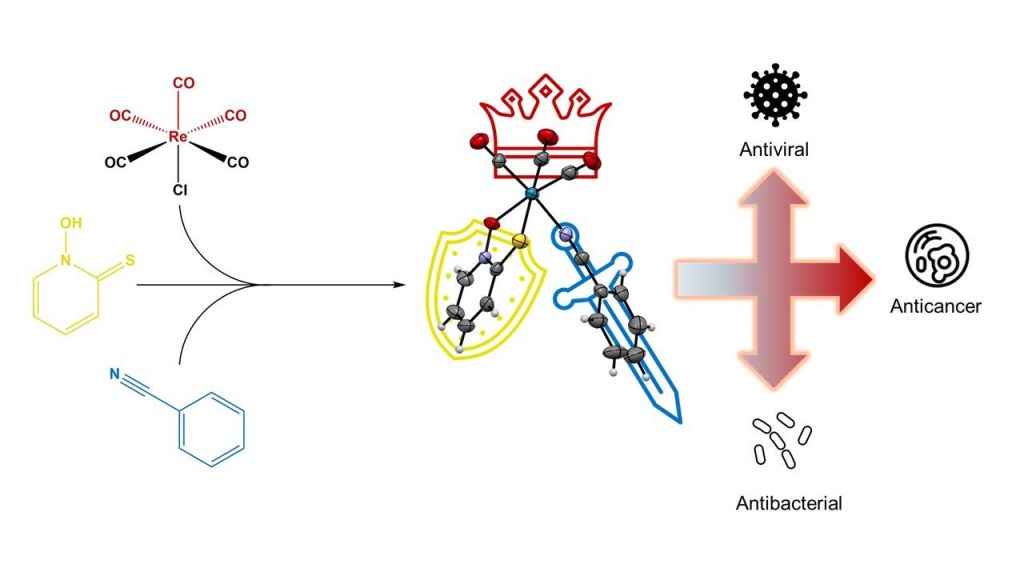
Recently, we reported the synthesis of the fac-tricarbonylrhenium(I) complex with a pyrithione ligand, which proved to be more challenging than initially expected [1]. During this study, we also identified several by-products and decomposition products, which we successfully characterised (see Scheme below). The stable fac-[Re(CO)3(pth)(benzonitrile)] complex was further investigated for its solution chemistry and evaluated for anticancer, antibiotic, and antiviral activities in collaboration with Professor Éva A. Enyedy’s research group.

Furthermore, in collaboration with Professor Janko Kos and Assistant Professor Ana Mitrović, we investigated the potential mechanism of action of inhibiting cysteine cathepsins, enzymes known to promote tumour growth. In addition, in partnership with Professor Stanislav Gobec and Assistant Professor Anže Meden, we conducted in silico studies to explore how this complex interacts with cathepsin B through docking and molecular dynamics simulations. The complex demonstrated promising anticancer activity against various cancer cell lines, antibiotic activity against different bacterial strains and notable antiviral activity against Herpes Simplex Virus 2. This is the first publication resulting from the doctoral research of Ph.D. student Uroš Rapuš.
More details can be found in the full publication that was published in ACS Omega:
1) U. Rapuš, T. Pivarcsik, A. Mitrović, J. Kljun, A. Bogdanov, G. Spengler, A. Meden, S. Gobec, J. Kos, E. Enyedy, I. Turel: Challenges in the preparation of a fac-tricarbonylrhenium(I) complex with pyrithione, its physico-chemical characterization and assessment of biological effects. ACS Omega, 10, 38272-38291, 2025.
https://doi.org/10.1021/acsomega.5c06647
2) T. Pivarcsik, M. A. Kiss, U. Rapuš, J. Kljun, G. Spengler, É. Frank, I. Turel, É. A. Enyedy: Organometallic Ru(II), Rh(III) and Re(I) complexes of sterane-based bidentate ligands: Synthesis, solution speciation, interaction with biomolecules and anticancer activity. Dalton Trans., 53, 4984-5000, 2024.
https://pubs.rsc.org/en/content/articlelanding/2024/dt/d3dt04138g
3) T. Pivarcsik, J. Kljun, S. Clemente Rodriguez, D. Cortéz Alcaraz, U. Rapuš, M. Nové, E. F. Várkonyi, J. Nyári, A. Bogdanov, G. Spengler, I. Turel, E. Enyedy: Structural and solution speciation studies on fac-tricarbonylrhenium(I) complexes of 2,2’-bipyridine analogues. ACS Omega, 9, 44601, 2024.
Fighting the SARS-CoV-2 Virus with Zinc Compounds
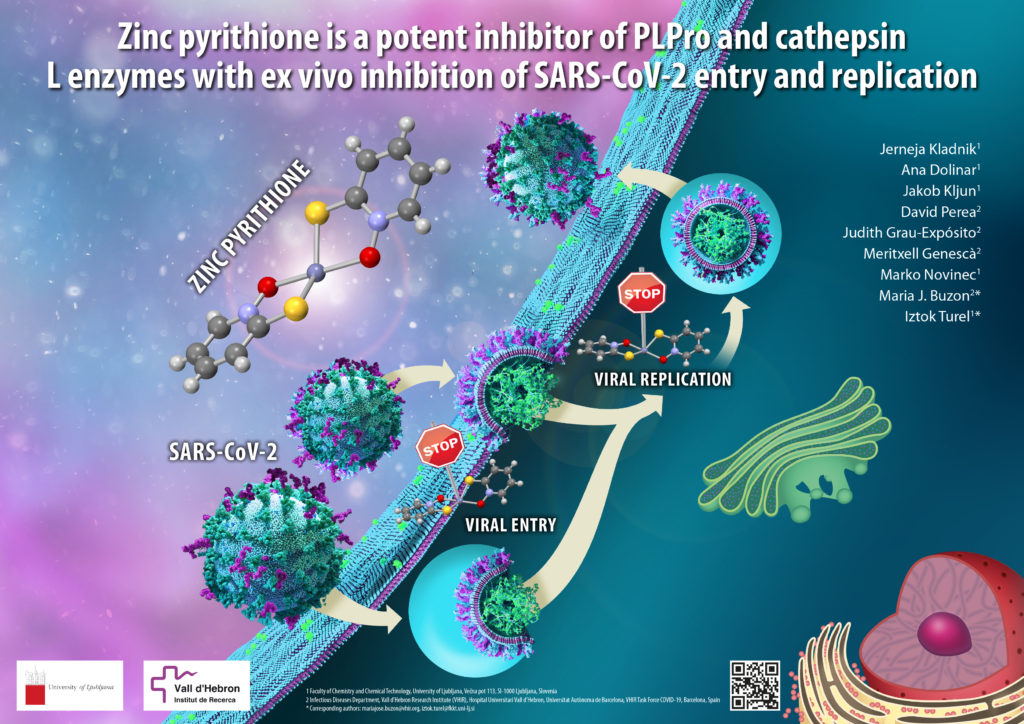
Over the past two and a half years, the SARS-CoV-2 coronavirus has greatly affected all of our lives. In addition to the rapid development and approval of various vaccines, research has also been conducted on therapeutic options to treat the COVID-19 disease. Oral medications that patients could simply use at home are especially desirable. We have prepared and tested zinc compounds that have proven to be very effective against the SARS-CoV-2 virus. The results are also interesting because one of the compounds tested, zinc pyrithione, has been known for a long time and has been shown to have antimicrobial properties (it is used in shampoos to treat dandruff caused by fungi of the Malassezia genus). Studying the use of already known agents to treat other diseases is currently a very popular strategy, as it can significantly shorten the drug approval process.
Zinc is an essential element vital for the functioning of humans and other living organisms and is involved in many biological processes. During the COVID-19 pandemic, much of the research focused on studying the importance of this element or, more specifically, zinc compounds. Zinc was also found to be important in the functioning of the SARS-CoV-2 virus. The researchers found that some zinc compounds effectively inhibit the enzymes cathepsin L and PLPro, which allow the virus to enter cells and replicate, respectively. Based on these encouraging results, our Spanish collaborators confirmed the highly potent antiviral activity of zinc pyrithione in an ex vivo system derived from primary human lung tissue. The results of this collaborative research were recently published in the Journal of Enzyme Inhibition and Medicinal Chemistry, and further research in this area is ongoing. The discovery of the inhibitory effect of zinc compounds on coronavirus is important to better understand how the virus works and to facilitate the development of potential zinc antiviral drugs. News of this discovery has also been reported on national television [https://365.rtvslo.si/arhiv/prvi-dnevnik/174893819] (appears between 5:40-7:40 min) and elsewhere.
Five scientists from our Faculty and four from Spain were involved in the research: Jerneja Kladnik, Ana Dolinar, Jakob Kljun, David Perea, Judith Grau-Expósito, Meritxell Genescà, Marko Novinec, Maria J. Buzon and Iztok Turel. This study, which lasted just over a year, was financially supported by the Slovenian Research Agency (ARRS).
The figure shows zinc pyrithione inhibiting the entry of SARS-CoV-2 virus into the cell and its replication (illustration by academy-trained painter Simon Kajtna).
Link to the article: https://www.tandfonline.com/doi/full/10.1080/14756366.2022.2108417.
Krka Grand Prize
Every year the well-established Slovenian pharmaceutical company Krka opens the call for applications for the Krka Prize contest. This year a record number of 114 undergraduate and postgraduate students responded to the call and among them 5 Krka Grand Prizes for special research achievements, 25 Krka Prizes and 44 Krka Prizes with special recognition were awarded to the students.
Turel Group is proud to announce that among the applicants, our postdoctoral researcher Dr. Jerneja Kladnik was awarded the Krka Grand Prize for Special Research Achievement for her PhD thesis under the supervision of Prof. Dr. Iztok Turel, in which she focused on the synthesis of biologically active metal complexes with pyrithione and its analogues. Turel Group congratulates Jerneja on this award and wishes her lots of success in her future research!
Successful end of »coronavirus« year 2020 and start of a new one in Turel group
Although coronavirus is still limiting and regulating our daily lives as well as affecting research activities, several positive things worth mentioning happened in our group in the last months of 2020.
In October 2020, a new PhD student (junior researcher) Simona Gričar has started her doctoral studies under the supervision of assist. prof. Jakob Kljun. Her study is funded by ARRS (Slovenian Research Agency) for four years and during this time she will be working on synthesis and evaluation of novel inhibitors of pharmaceutically relevant metalloproteins. Simona successfully finished her Master thesis in our group at FCCT in 2019. After the defense, she was employed for one year at the Faculty of Pharmacy in Ljubljana working on a project researching the role of immunoproteasome in immune response.
In December 2020, a member of our group Jerneja Kladnik also finished her doctoral study. The Turel group thanks and congratulates Jerneja for her successful defense of PhD thesis entitled »Synthesis and biological activity of coordination compounds of pyrithione analogues«. Like many other events in 2020, the dissertation was defended online and Jerneja did an excellent job!
We are also very proud to have obtained funding from our national research agency (ARRS) for a project on Metallo-therapeutics to combat Covid-19 disease. This funding will enable researchers dr. Jerneja Kladnik, dr. Ana Dolinar and MChem. Maša Masič to study new potential effective therapeutic strategies against SARS-COV-2 in 2021.
The Turel group welcomes new team members and hopes for their pleasant and productive stay.
Metal based compounds as drugs against many diseases
People were using metals and metal compounds for the treatment of various diseases for millennia. Nowadays chemists rationally synthesise metal complexes with the aim to use them in medical practice.
Without any doubt, the most well-known drug of this type is cisplatin which is a famous anticancer drug. In our labs the most efforts were put in the research of novel anticancer agents, mostly based on ruthenium, which was frequently described in our previous posts.
However, my research career started by investigating metal complexes of antiviral drug acyclovir (acv). My very first paper was on coordination chemistry of a copper(II)-acv complex [1]. Later, several isolated copper-acv complexes were tested for antiviral activity (Herpes Simplex Virus, HSV) but results were not very promising [2]. In 2020 coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shocked us all. Due to the COVID-19 pandemic, many researchers are now trying to find a drug (or suitable methods) to treat this disease. It is also promising that funding options for coronavirus research appeared very quickly and worldwide research consortia are working towards the common goal. We are also considering joining the effort though very quick solutions are not very probable in this field of research.
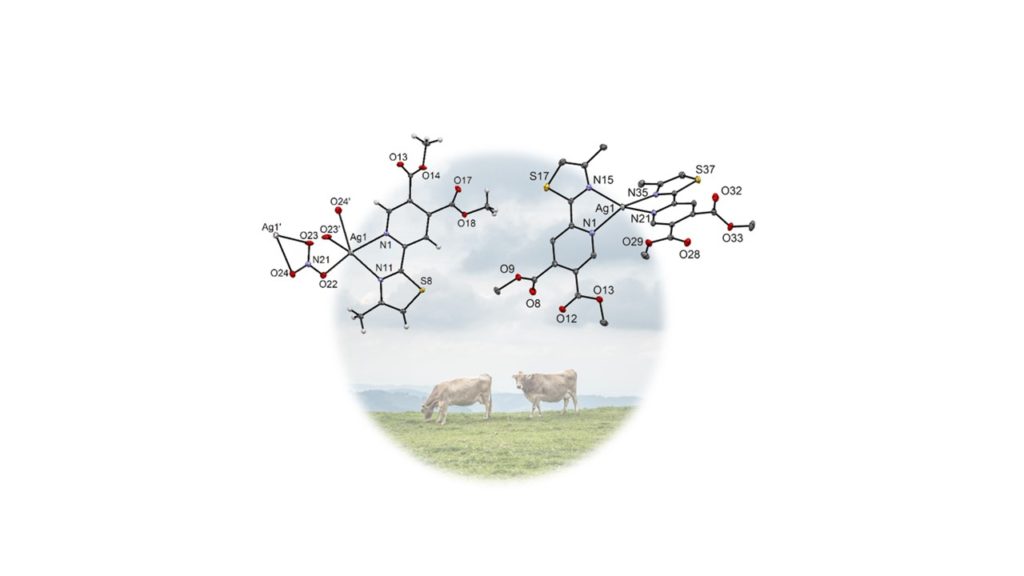
In previous years, our metal complexes were also tested for antibacterial, antifungal and other antimicrobial activities [3-5]. Our last contribution in this direction is a recently accepted joint paper (cooperation with groups of Jasmina Nikodinovic-Runic and Milos Djuran from Serbia) accepted in Dalton Transactions. In this paper we have studied silver complexes with pyridine-4,5-dicarboxylate type of ligands. Infections of the cow udder leading to mastitis and lower milk quality are one of the biggest problems in the dairy industry worldwide. Unfortunately, therapeutic options for the treatment of cow mastitis are limited as a consequence of the development of pathogens that are resistant to conventional antibiotics. Therefore, new antibacterial agents are needed and our silver complexes have so far shown promising results.
We all hope that funding agencies will now be encouraged to more strongly support the research of novel antimicrobial compounds. For example, it is well-known that experts are warning about superbugs, bacteria that are resistant to most available antibacterial agents [6]. It would surely not be good to wait so unprepared for something to happen as in the case of coronavirus.
- B. Blažič, I. Turel, N. Bukovec, P. Bukovec and F. Lazarini, Synthesis and Structure of Diaquadichlorobis 9-(2-Hydroxyethoxy)-Methyl Guanine Copper(II), J. Inorg. Biochem., 51, 737 -744 (1993).
- M. Panteva, T. Varadinova, I. Turel, Effect of Copper Acyclovir Complexes on Herpes Simplex Virus Type 1 and Type 2 (HSV-1, HSV-2) Infection in Cultured Cells, Metal Based Drugs, 5, 19-23 (1998).
- I. Turel, L. Golič, P. Bukovec, M. Gubina, Antibacterial tests of Bismuth(III)-Quinolone (Ciprofloxacin, cf) Compounds Against Helicobacter pylori and Some Other Bacteria. Crystal Structure of [(cfH2)2 Bi2Cl10] .4H2O, J. Inorg. Biochem., 71, 53-60 (1998).
- J. Kljun, I. Bratsos, E. Alessio, G. Psomas, U. Repnik, M. Butinar, B. Turk, I. Turel, New uses for old drugs: attempts to convert quinolone antibacterials into potential anticancer agents containing ruthenium, Inorg. Chem., 52, 9039–9052 (2013).
- J. Kljun, A. J. Scott, T. Lanisnik Rizner, J. Keiser, I. Turel, Synthesis and biological evaluation of organoruthenium complexes with azole antifungal agents. First crystal structure of a tioconazole metal complex, Organometallics, 33, 1594−1601 (2014).
- R. Rappuoli, D. E. Bloom, S. Black, Deploy vaccines to fight superbugs, Nature, 552, 165-167 (2017).
Ruthenium anticancer complexes. Looking for possible targets and further insights into the mechanisms of action.
This year we have continued with research on versatile ruthenium compounds and several publications were successfully published.
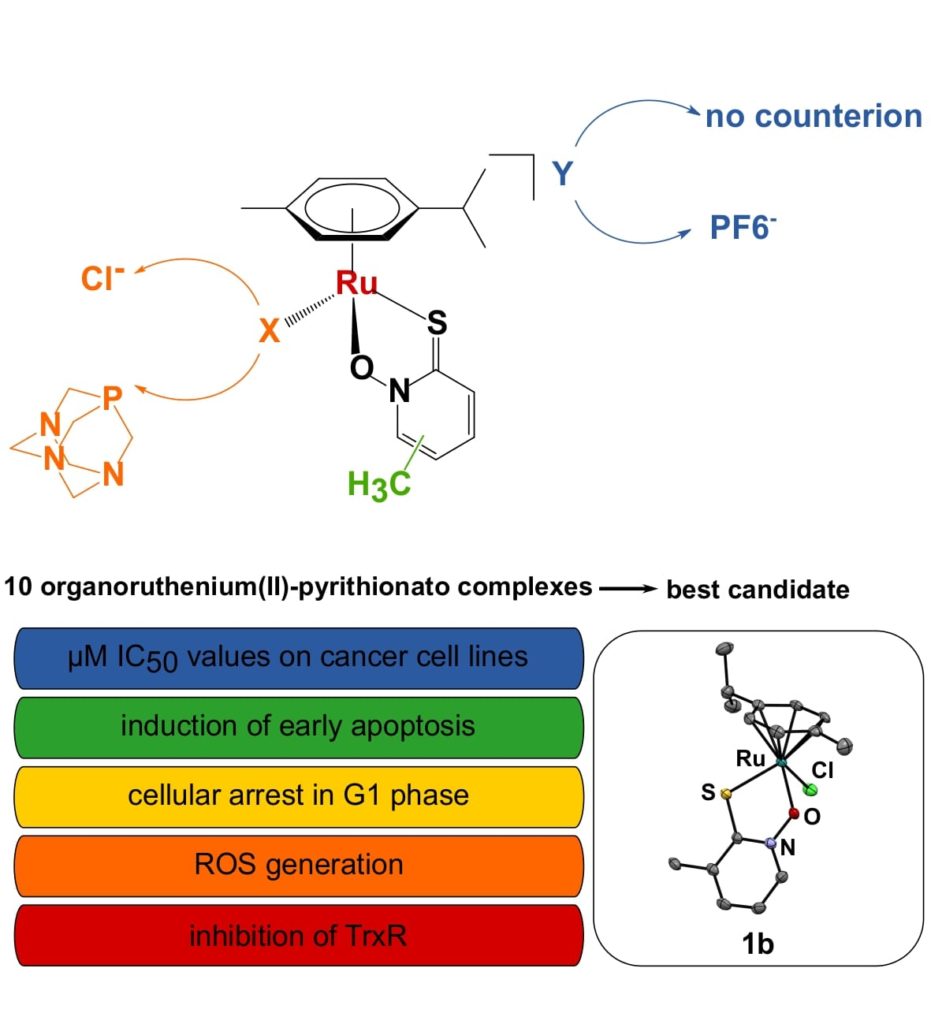
Promising research on pyrithione complexes was continued within doctoral study of Jerneja Kladnik. Several novel pyrithione analogues were prepared and characterized, extensive biological tests were performed in the cooperation with dr. I. Romero-Canelón (University of Birmingham, UK) and the group of Prof. I. Ott (Technical university Braunschweig, Germany). The aim of the study was the fine-tuning of the anticancer activity through structural alterations of organoruthenium(II) complexes with pyrithiones.
Results were published in Chemistry – A European Journal: Towards Identification of Essential Structural Elements of Organoruthenium(II)-Pyrithionato Complexes for Anticancer Activity, https://doi.org/10.1002/chem.201903109
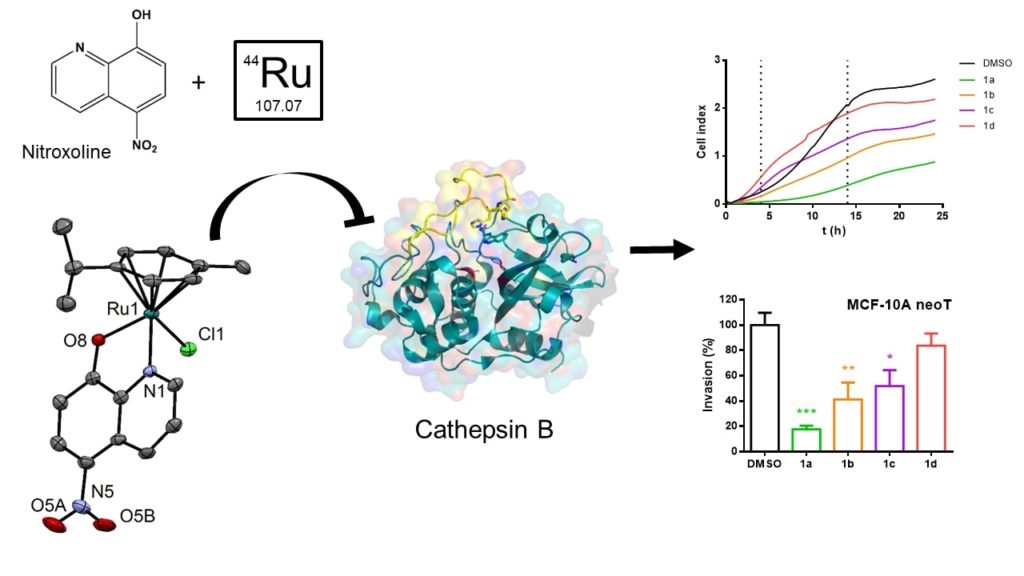
Further, two studies involved research on ruthenium complexes with 8-hydroxyquinolone (8-HQ) ligands. The first was performed in cooperation with our Slovenian colleagues from the Faculty of Pharmacy and Jožef Stefan institute (groups of Prof. S. Gobec and Prof. J. Kos). Eleven ruthenium compounds bearing either clinical agent nitroxoline, which was previously identified as potent selective reversible inhibitor of Cathepsin B (catB) activity, and its derivatives were prepared. We have demonstrated that organoruthenation is a viable strategy for obtaining highly effective and specific inhibitors of catB endo- and exopeptidase activity as shown by using enzyme kinetics and microscale thermophoresis.
Results were published in Inorganic Chemistry: Organoruthenated Nitroxoline Derivatives Impair Tumor Cell Invasion through Inhibition of Cathepsin B Activity,
https://pubs.acs.org/doi/10.1021/acs.inorgchem.9b01882
The second study with 8-HQ ligands was performed in the cooperation with colleagues from the National University of La Plata, Argentina (Ana L. Di Virgilio and Ignacio E. Leon). This was a comparative antitumor study of organoruthenium complexes with two selected 8-hydroxyquinolines (5-bromo and 5,7-dibromo substituted) on 2D and 3D cell models of bone, lung and breast cancer. In addition to both compounds potently inhibiting the cell invasion of multicellular spheroids, the monosubstituted compound exhibited also interesting antimetastatic action.
Results were published in Metallomics:
- C. Ruiz, J. Kljun, I. Turel, A. L. Di Virgilio, I. E. Leon, Comparative antitumor studies of organoruthenium complexes with 8-hydroxyquinolines on 2D and 3D cell models of bone, lung and breast cancer, Metallomics, 11, 666-675 (2019).
The next ligands with tradition in our group are b–dikenonates and in the newest study we joined the forces with the group of Jana Roithova (Radboud University, Nijmegen, Netherlands; previously Charles University, Prague). The knowledge on the interactions of metal-based drugs with biomolecules is extremely important but also difficult to study. In this paper the interactions of anticancer organoruthenium(II) complexes with biologically important sulfur containing molecules – cysteine, N-acetylcysteine and glutathione – were studied by mass spectrometry and infrared photodissociation spectroscopy.
Results were published in the Dalton Transactions:
- Briš, J. Jašík, I. Turel and J. Roithová, Anti-cancer organoruthenium(II) complexes and their interactions with cysteine and its analogues. A mass-spectrometric study, Dalton Transactions, 48, 2626–2634 (2019).
Our research also involved sulfonamide ligands that are well known medicinal agents. It is not easy to find the targets of metal-based drugs but enzymes are always high on the list. We have found that organoruthenium(II) complexes of sulfonamide acetazolamide potently inhibit human carbonic anhydrase isoforms. This study was performed in the cooperation with Claudiu T. Supuran (University of Florence, Italy).
Results were published in the Journal of Enzyme Inhibition and Medicinal Chemistry:
- Seršen, K. Traven, J. Kljun, I. Turel*, C. T. Supuran*, Organoruthenium(II) complexes of acetazolamide potently inhibit human carbonic anhydrase isoforms I, II, IX and XII, J. Enzyme Inhib. Med. Chem., 34, 388-393 (2019).
A study involving ruthenium complexes from our library of compounds (with 8-hydroxyquinolone and b–-dikenonate ligands) was conducted in cooperation with the group of dr. Yangzhong Liu (University of Science and Technology of China, Hefei, China). The main aim was to establish the type of interactions between zinc finger protein NCp7 and selected ruthenium compounds. Proteins from this group are also explored as possible targets in the design of novel HIV drugs. Inhibitors of NCp7 frequently provoke the release of zinc from the protein. We found that the type of interaction is strongly dependent on the type of ligand bound to the metal center.
Results were published in Chemistry – A European Journal: Covalent versus Non‐covalent Binding of Ruthenium η6‐p‐Cymene Complexes to Zinc‐finger Protein NCp7, DOI: https://doi.org/10.1002/chem.201902434
Last but not least – a nice study was also conducted with colleagues from Croatia, led by dr. Srećko Kirin and dr. Anamaria Brozović (Ruđer Bošković Institute). The synthesis, characterization, and biological properties of organometallic ruthenium(II)-arene complexes with triphenylphosphine amino acid bioconjugates were thoroughly studied.
Results were published in Bioorganic chemistry:
- Pernar, Z. Kokan, J. Kralj, Z. Glasovac, L.-M. Tumir, I. Piantanida, D. Eljuga, I. Turel, A. Brozovic, S. I. Kirin, Organometallic ruthenium(II)-arene complexes with triphenylphosphine amino acid bioconjugates: Synthesis, characterization and biological properties, Bioorganic Chemistry, 87, 432-446 (2019).
150th anniversary of the periodic table of chemical elements. Periodic table, Mendeleev and philately.
Russian chemist Dmitri I. Mendeleev (1834–1907) has formulated the periodic system of chemical elements (PSCE) in 1869. Frequently, this is considered as one of the greatest inventions in chemistry. The year 2019 marks the 150th anniversary of this discovery and has thus been proclaimed the “International Year of the Periodic Table of Chemical Elements (IYPT2019)” by the United Nations General Assembly and UNESCO.
Philately is my hobby and in the last years I have focused especially on chemical items. From time to time I prepare short articles for newspapers or journals on various topics and support them with philatelic materials (1-4). I have recently prepared a short contribution about the periodic table, Mendeleev and also about some lastly discovered chemical elements for Slovenian journal »Kemija v šoli in družbi« (Chemistry in school and society) (5). Therein I have involved selected philatelic items that are related to periodic table and some I present also below. I am sure that this year some new stamps, first day covers (FDC), seals, etc., will appear to celebrate IYPT 2019.
Figure 1-6 (from top to bottom):
It is understandable that most of stamps that show Mendeleev were issued in Russia (and previously in Soviet Union). To the best of my knowledge, the first stamps showing Mendeleev were issued in 1934 (100 years after his birth).
Fig. 1 shows one of these. The stamp issued to commemorate 100 years of periodic table of chemical elements discovery (1969) shows Mendeleev deep in thought (Fig. 2). Two chemical elements that represented some problems to Mendeleev are marked in red. Gallium was then not yet discovered but he has predicted its existence. It was discovered by French chemist Paul Emile Lecoq de Boisbaudran in 1875. In contrast, indium was already known but its relative atomic mass was not determined accurately. Non-accurately determined relative atomic masses were the reason that in the first versions of the periodic table some elements were placed in wrong positions which was later corrected in improved versions. Interestingly, also the available relative atomic mass of ruthenium, the most important element for our research group was not very precise at that time (104.4; correct value is 101.1).
A commemorative postcard (Fig. 3) showing the portrait of Mendeleev with a stamp and corresponding seal were issued in 1957 (50 years after his death).
A souvenir sheet from 1969 (100 years of PSCE discovery) shows the stamp with Mendeleev which is embedded in his signed and dated work paper (Fig. 4).
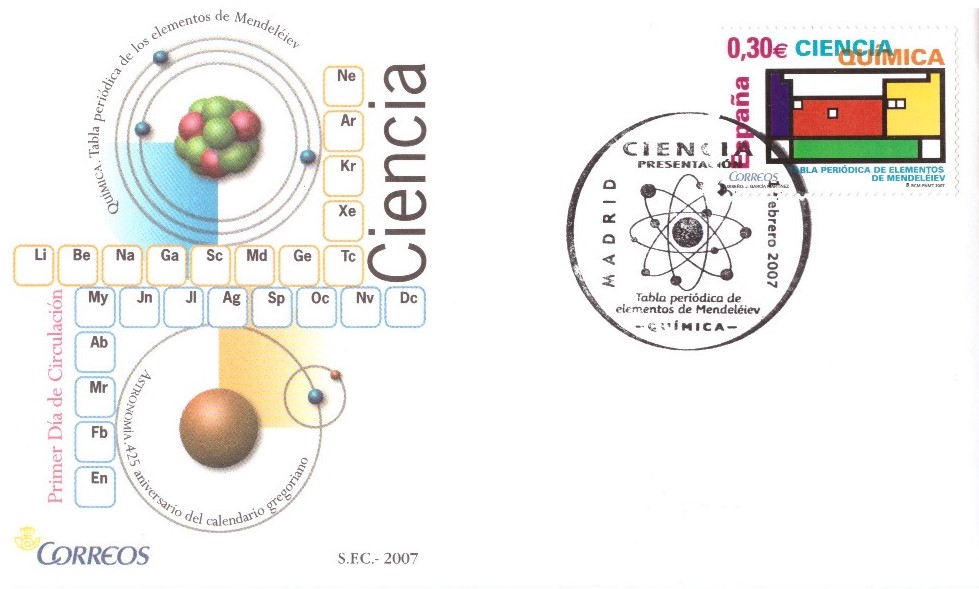
Spanish post has issued the first day cover in 2007 (hundred years after Mendeleev died) (Fig. 5). The corresponding stamp shows a colourful drawing of the periodic table which was inspired by the paintings of Piet Mondrian, the Dutch painter. Four smaller white squares in the periodic table represent elements that were predicted by Mendeleev (scandium, technetium, gallium, and germanium).
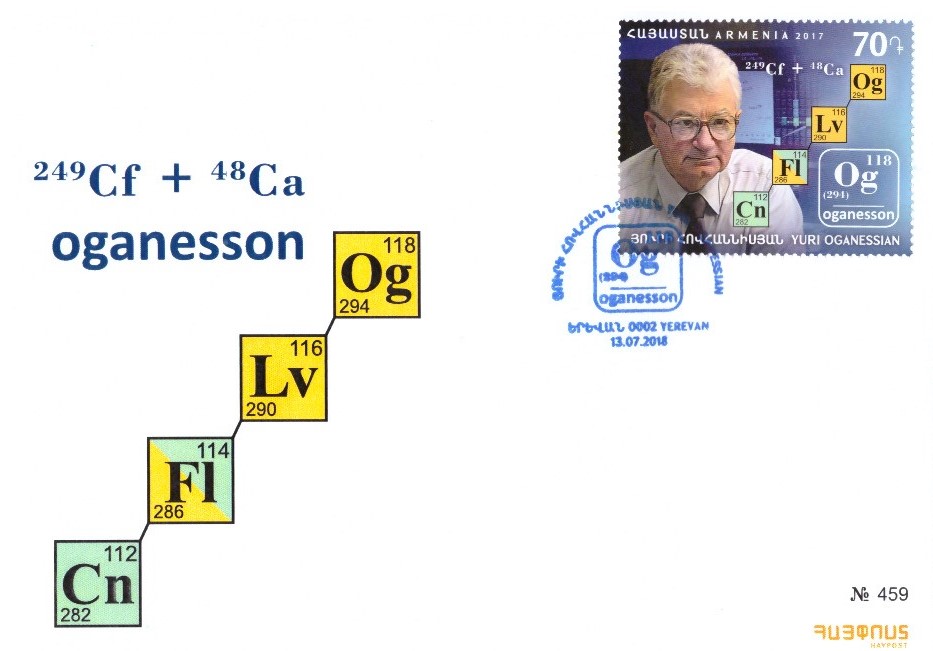
First day cover from Armenia shows Yuri Oganessian who is one of the leading nuclear physicists in the field of superheavy chemical elements (Fig. 6). He was involved in the preparation of several elements, among them is also the currently heaviest which is named oganesson (Og) in his honour. Artificial heavy elements are very unstable and the radioactive decay pathway of the isotope oganesson-294 is also shown on the stamp and cover. With the isolation of this element the 7th row of periodic table was completed (Z = 118). The methods of how to “synthesize” new chemical elements, how to verify and name them, as well as some speculations about possible new element discoveries in the coming years were nicely presented in the paper of Prof. J. Reedijk recently (5).
References:
- I. Turel, Acta Chim. Slov., 59, p. S94-S96 (2012).
- I. Turel, Acta Chim. Slov., 60, S115-118 (2013).
- D. Rabinovich, I. Turel, Philatelia chimica et physica, 35, 171-173 (2014).
- I. Turel, Kemija v šoli in družbi, nr. 1, p. 1-4 (2019).
- J. Reedijk, Row 7 of the periodic table complete: Can we expect more new elements; and if so, when?, Polyhedron, 2018, vol. 141, str. 1–4.
Our study on inhibition of cholinesterases and glutathione-S-transferases by organoruthenium complexes selected for cover feature in October 2018 edition of ChemMedChem
In this paper , a library of 17 organoruthenium compounds was screened for inhibitory activity against cholinesterases and glutathione-S-transferases of human and animal origins. The cover feature picture (see below) shows an organoruthenium-pyrithione complex which inhibits cholinesterases and glutathione-S-transferases at pharmaceutically relevant concentrations while we observed no undesirable physiological responses on the neuromuscular system as well as no toxicity against non-transformed cells. Combining these results with our previous study (Kljun et al. Dalton Trans. 2016, 45, 11791-11800) which has shown the ability of this compound to inhibit enzymes involved in hormone-dependant breast cancer as well as high toxicity on MCF-7 breast cancer cell line it is supposed that the cover page compound can be an interesting candidate as a multi-target drug for the treatment of cancer or Alzheimer’s disease. In this multidisciplinary study we have joined the forces with our colleagues from different faculties of the University of Ljubljana (Biotechnical Faculty, Faculty of Medicine, Veterinary Faculty) and a student from University of Rijeka was also involved.
Cover page of paper:
S. Ristovski, M. Uzelac, J. Kljun, T. Lipec, M. Uršič, Š. Zemljič Jokhadar, M. C. Žužek, T. Trobec, R. Frangež, K. Sepcic, I. Turel, Organoruthenium prodrugs as a new class of cholinesterase and glutathione-S-transferase inhibitors, ChemMedChem, 13, 2166-2176 (2018).
https://onlinelibrary.wiley.com/toc/18607187/2018/13/20
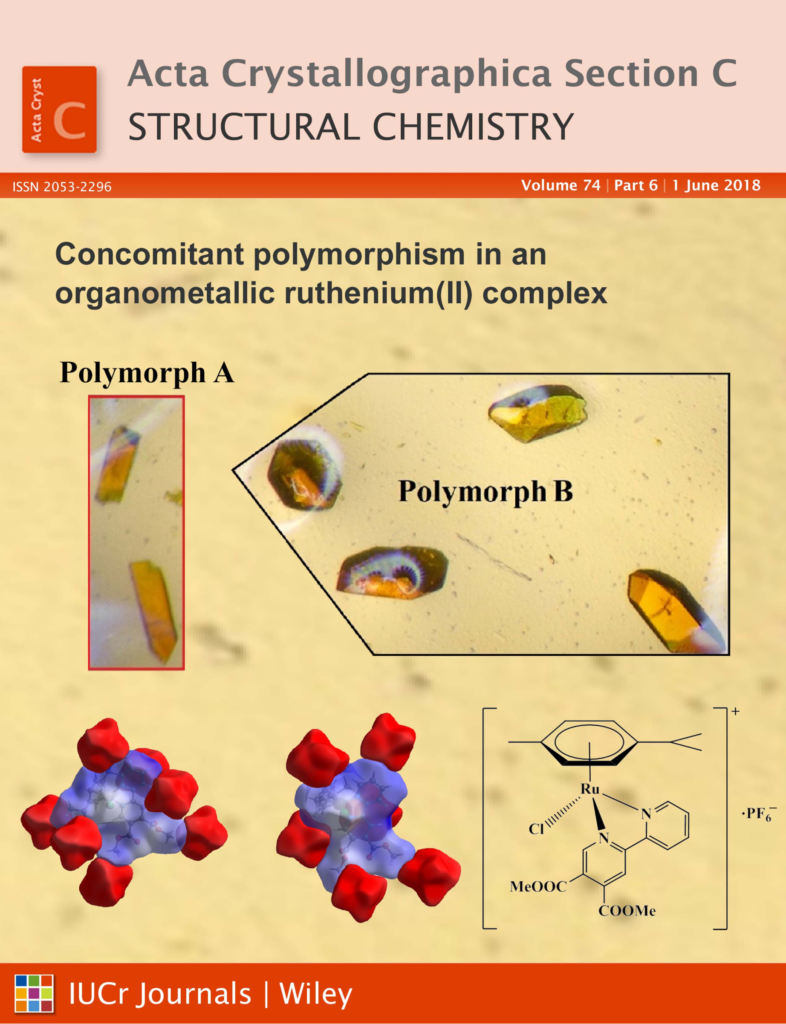
Our study on concomittant polymorphism selected for cover page in June 2018 edition of Acta Crystallographica, Section C
Polymorphism is a well-known phenomenon and represents the ability of a solid material to exist in more than one form. It is not only important from the academic point of view but has also many practical applications or consequences. A typical example is the pharmaceutical industry where many drugs receive regulatory approval for only one polymorphic form. However, there are known examples where the patent was later obtained also for different form.
Sometimes the growth rates of polymorphic forms are sufficiently similar that such crystals coexist. In such case we call them concomitant polymorphs.
Concomitant polymorphism is rarely mentioned for ruthenium complexes and we have previously never observed it in any of previously synthesized compounds in our lab. However, during her Ph.D. study, Katja Traven has prepared several organometallic ruthenium(II) complexes, among other also with dimethyl 6-(pyridine-2-yl)pyridine-3,4-dicarboxylate ligand. In the latter system two types of crystals were observed in the reaction mixture and studied in cooperation with the Slovak group led by Prof. J. Kozisek. Crystal structures of both polymeric forms have shown that the most bond lengths are approximately the same but differences in the orientaion of methoxycarbonyl groups and p-cymene were found. These concomitant polymorphs also differ in melting point temperatures for cca. 10 °C.
Cover page of paper: K. Traven, I. Turel*, J. Koziskova, L. Bučinský, J. Kozisek*, Concomitant Polymorphism in Organometallic Ruthenium(II) Complex with N,N-donor Ligand, Acta Crystallogr. C74, 683-689 (2018).
Spring in Ru lab
Several interesting and important things have happened in our group during spring 2018.
During his short stay at our Faculty, Prof. Bostjan Kobe has shortly visited our labs. Bostjan and Iztok were classmates during their study of chemistry at the Univerity of Ljubljana. Bostjan later went to University of Texas Soutwestern America Medical Center at Dallas, USA, for his Ph.D. studies under the supervision of Nobel prize winner Johann Deisenhofer and he is now Professor of Structural Biology at the University of Queensland in Australia.
It is always nice to meet old friends to talk about the past, present and also future!

Professor Roger Alberto from the University of Zürich, Switzerland has visited our labs in May and held a lecture titled »Bioorganometallic Technetium and Rhenium Chemistry: Theranostics, Fundamentals and Applications« for the students of the FKKT doctoral study programme. Complexes of the radioactive element technetium are well established in diagnostic nuclear medicine, and various complexes of the gamma-emitting nuclide 99mTc are routinely used for organ imaging (heart, brain, etc). Professor Alberto was involved in the discovery of compound MIP 1405 which is on a good way to enter clinical use soon.
In spring 2018 two members of the group have finished their doctoral study. The Turel group thanks and congratulates to Matija Uršič on his successful defence of PhD thesis entitled »Synthesis and characterisation of ruthenium complexes with phosphine ligands« and to Katja Traven (nee Krančan) on her successful defence of PhD thesis with the title »Novel ruthenium coordination compounds with N,N-, N,O- and N,N,N-donor ligands«. We wish both of them all the best and continued success with new challenges.